Sucrose is a molecule composed of two monosaccharides, namely glucose and fructose. This non-reducing disaccharide has a chemical formula of C12H22O11.
Sucrose is commonly referred to as table sugar or cane sugar.
In a C12H22O11 molecule, the fructose and glucose molecules are connected via a glycosidic bond. This type of linking of two monosaccharides called glycosidic linkage. Sucrose has a monoclinic crystal structure and is quite soluble in water. It is characterized by its sweet taste.
William Miller, an English chemist, coined the word sucrose in the year 1857. It is widely used as a sweetener in food. C12H22O11 can be obtained from sugar beets or sugar canes, but it must be refined to be fit for human consumption. Refined sucrose (or sugar) is a popular ingredient in many food recipes because of its sweet taste.
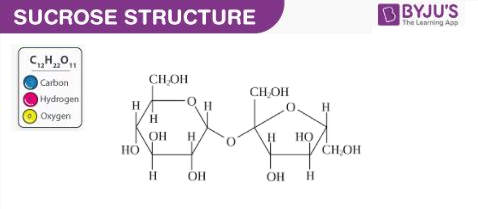
As discussed earlier, sucrose is a disaccharide which is made up of two monosaccharides. The structure of a sucrose molecule is illustrated below.

The glycosidic linkage that connects the two carbohydrate groups can be observed in the illustration provided above. There are no anomeric hydroxyl groups in a sucrose molecule. It can, therefore, be classified as a non-reducing sugar (since it does not act as a reducing agent).
| Chemical Formula of Sucrose | C12H22O11 |
| Molar Mass or Molecular Weight | 342.30 g/mol |
| Density | 1.587 g/cm 3 |
| Physical Appearance | White, crystalline solid |
| Melting Point | Decomposes at 459 K |
When heated to temperatures above 186 degrees Celsius, sucrose undergoes a decomposition reaction to give rise to caramel. In a manner that is similar to other carbohydrates, sucrose undergoes combustion in the presence of oxygen to yield water and carbon dioxide as the products. It can also be noted that sucrose can be reacted with potassium nitrate (a powerful oxidizing agent with the chemical formula KNO 3 ) to yield a special type of fuel known as rocket candy. The chemical equation for the reaction between sucrose and potassium nitrate is provided below.
It can also be noted that sucrose undergoes a combustion reaction with chloric acid to yield hydrochloric acid, water, and carbon dioxide. This reaction can be represented by the following chemical equation:
Sucrose can be subjected to dehydration in the presence of sulfuric acid in order to obtain a black solid that is rich in carbon. The idealized chemical equation for this process is provided below.
C 12 H 22 O 11 + H 2 SO 4 → 11H 2 O + 12C (carbon-rich solid) + heat
It can also be noted that small quantities of SO 3 can be produced in this process.
Some of the important uses of this compound are listed below.
Thus, the structure of a sucrose molecule, its physical and chemical properties, and its uses are discussed briefly in this article. To learn more about this disaccharide and other disaccharides, such as lactose, register with BYJU’S and download the mobile application on your smartphone.
Sucrose is a disaccharide sugar which means that it consists of two units of monosaccharide sugar. The two units are glucose and fructose, for sucrose. The name saccharose is derived from the French word fruit.
Sucrose is used in foods and soft drinks as a sweetener, in syrup processing, in invert sugar, confectionery, preserves and jams, demulcent, medicinal products, and caramel. Sucrose is also a chemical carrier for detergents, emulsifiers, and other derivatives of saccharose.
Sucrose is found in fruits and vegetables, and is processed for use in cooking and food processing from sugar cane and sugar beets. The sucrose found naturally in sugar cane, sugar beets, bananas, grapes, carrots, and other fruits and vegetables in your sugar bowl is the same sucrose.
Sugar or sucrose is only slightly soluble in ethanol. In addition, if the alcohol is cold it will dissolve even less of the sucrose. The sugar not dissolving within the ethanol settles at the bottom of the bottle. The salt is also very water-soluble.
Sucrose is the most common type of carbohydrate used for the carriage of carbon in a plant. Sucrose can be dissolved in water, thus retaining a stable structure. Sucrose will then be transported into the phloem by plant cells, the special vascular tissue intended for sugar transport.